What Is the Hybridization of the Central Atom in Xef4
XeF 4 consists of two lone pair electrons. The structure of the krf 2 molecule is linear with krf distances of 1889 pm.

Hybridization Of Xef4 Xenon Tetrafluorid Youtube
Now X quotient remainder2426.

. Which compound has square pyramidal shape. In the formation of XeF 4 two of the 5p orbital electrons which in the excited state move to fill the vacant 5 d orbitals. It is square planar.
In this case if we gaze upon the valence shell of Xe the total amount of electrons is six in the 5p orbital as well as two electrons in the 5s orbital. Sp3d2 With 6 electron domains around the central Xe atom it will need 6 hybrid orbitals and therefore need to combine 6 orbitals to make the hybrids. What is the hybridization of the central atom in.
What is the hybridization of the central atom in XeF4. Hybridization What are the approximate bond angles in this substance. Sp2 sp3 dsp3 sp3d2.
Its hybridization is sp 3 its shape is pyramidal. Bond angles _____ Question. Now looking on the table described above hybridization of XeF4 sp3d2.
It is distorted octahedral because of the lone pair present. For example trigonal bipyramid geometry can lead to 3 different bond angles. Now looking on the table described above hybridization of XeF4 sp3d2.
What is the hybridization of the central atom in XeF4. Hybridization What are the approximate bond angles in this substance. The hybridization in Xenon is sp 3 d 2 because there is a migration of two electrons of p to d orbital which results in the formation of sigma bond with F.
Quantum mechanics explains the idea of hybridization. Hybridization of XeF4 Xenon Tetrafluoride. Bond angles B.
Hybridization What are the approximate bond angles in this substance Bond angles Question. What are the approximate bond angles in this substance. Now 368 quotient 4remainder 4 Remainder 2422.
1 The hybridization of the central atom in the XeF4 molecule is. Is xef4 pyramidal. Xenon Tetrafluoride consists of the central atom xenon which is the epicenter where the hybridization takes place.
Hybridization_____ What are the approximate bond angles in this substance. What is the hybridization of the central atom in. What is the hybridization of the central atom in XeF4.
What is the. What is the hybridization of the central atom in XeF. What are the approximate bond angles in this substance.
As we know in the case of XeF 4 or xenon tetrafluoride the hybridization of xeof₄ occurs in the central atom which is Xenon Xe. Solve any question of Chemical Bonding and Molecular Structure with-. Valence electron of Xe 8 that of F7 here no.
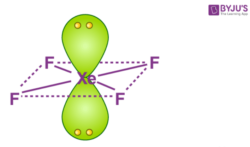
The central atom has 6 electron pairs out of which two are lone pair electrons. What is the hybridization of the central atom in XéF4. As a result there are 4 unpaired electrons which include 2 in 5p and 2 in 5d orbitals.
As we know the hybridization is determined by the steric number which is equal to the number of lone pairs and the sigma bondsThe central atom of Xe is having 4 sigma bonds and 2 lone pairs which implies the. What is the hybridization of the central atom in XeF4. Now we have to determine hybridization of XeF4.
What is the hybridization of the central atom in XeF4. What is the hybridization of the central atom in XeF4. Hybridization of XeF4.
What is the hybridization of the central atom in. Adding we get 82836. Now 368 quotient 4remainder 4 Remainder 2422.
In X e F 4 Xe is the central atom and it is s p 3 d 2 hybridized having 2 lone pair on it having square planar shape. Answer 1 of 12. Krypton tetrafluoride krf4 molar mass molecular weight.
Bond angles What is the hybridization of the central atom in BrCl3. Hybridization What are the approximate bond angles in this substance. Number of surrounding atoms 12 valence electrons of central atom - valency of surrounding atoms - charge For XeF4.
In X e F 4 Xe is the central atom and it is s p 3 d 2 hybridized having 2 lone pair on it having square planar shape. Krypton is the central atom in this case because it is the least electronegative of the two atoms involved as. Bond angles B.
If more than one bond angle is possible separate each with a space. Trigonal bipyramidal sp3d Video Explanation Answer XeF4 is AB4L2 in which the Xenon is bonded with four fluorine atoms with 4 sigma bonds and xenon. The easiest way to know the hybridisation is to use the formula.
Of F atom is 4 so total valence electron becomes 7428. What is the hybridization of the central atom in ClF3. 41284 42 This means 4 bond pairs and 2 bond pairs.
What is the hybridization of the central atom in XeF4. Some molecular compounds that adopt square pyramidal geometry are XeOF 4 and various halogen pentafluorides XF 5 where X Cl Br I. What is the hybridization of the central atom in xenon tetrafluoride.
Bond angles _____ B. Now we have to determine hybridization of XeF4. Octahedral geometry can lead to 2.
Adding we get 82836. This results in sp 3 d 2 hybridization. The central atom of Xe has 8 valance orbitals out of which 4 electrons form single bonds with 4F atom and the rest are the lone pairs.
The VSEPR predicts the Square Planar shape. Valence electron of Xe 8 that of F7 here no. Therefore Xe will combine 1 s.
Of F atom is 4 so total valence electron becomes 7428. The valence shell of the atom contains 6 electrons in the 5p orbital whereas the 5s orbital entails 2 electrons. It also represents the theory of valence bonds.
Now X quotient remainder2426. What is the hybridization of XeF4. It is the process where the orbitals of an atom fuse and form a new hybridized orbital to create the geometry of molecules along with distinguishing bonding properties.
Hybridization_____ What are the approximate bond angles in this substance. Solve any question of Chemical Bonding and Molecular Structure with-. XeF4 is d2sp3 hybridized and contains 2 lone pair and 4 bonding pairs of valence electrons around the Xenon.

Hybridization Of Xef4 Xenon Tetrafluorid Youtube
Hybridization Of Xef4 Hybridization Of Xe In Xenon Tetrafluoride

No comments for "What Is the Hybridization of the Central Atom in Xef4"
Post a Comment